Copyright © 2022 Foshan MBRT Nanofiberlabs Technology Co., Ltd All rights reserved.Site Map
In the current context of increasingly severe water shortages and water pollution problems, the development of efficient and stable sewage treatment technologies has become the focus of attention for global researchers. Membrane separation technology, with its advantages of energy conservation and environmental friendliness, shows great potential in the field of sewage treatment. However, the insufficient stability of traditional membrane materials in harsh environments has limited their wide application. Recently, an innovative study has successfully overcome the problems of pore structure optimization and hydrophilic modification of polytetrafluoroethylene (PTFE) nanofiber membranes, bringing new hope to sewage treatment technologies. The research results were published in the journal Separation and Purification Technology.
To prepare PTFE nanofiber membranes, an 8 wt% PEO aqueous solution needs to be prepared first. The dried PEO powder is stirred in deionized water at 80°C for 12 hours to make it fully dissolved. Then, according to the ratios specified in Table 1, this PEO solution is mixed with PTFE emulsion and stirred for 6 hours to obtain a homogeneous spinning solution. After preparation, the prepared spinning solution is injected into a commercial electrospinning device. Through 3 - hour electrospinning machine spinning, a PTFE/PEO precursor nanofiber membrane is obtained. Finally, the precursor membrane is heated at 380°C for 30 minutes in an air environment, and PTFE nanofiber membranes with different morphologies can be prepared.
The hydrophilic modification of the above - prepared PTFE nanofiber membranes is carried out as follows: First, the PVA powder is dissolved in deionized water at 90°C for 12 hours to obtain a 2 wt% PVA aqueous solution. Before coating the PVA solution, the PTFE nanofiber membrane is immersed in ethanol for pre - wetting treatment. After the membrane is completely dried, it is immersed in a 5 wt% dilute GA solution for the acetalization reaction. After the reaction is completed, the membrane is rinsed with deionized water to remove excess reagents and then dried to a constant weight. The membrane is numbered as M3 - x, where x (taking values of 0, 10, 20, and 30) represents different acetalization reaction times.

Figure 1. Hydrophilic Modification and Preparation of PTFE Nanofiber Membranes
(1) Preparation by the Continuous Spinning - Sintering - Coating Method
The research adopted an innovative continuous preparation process, namely the "continuous spinning - sintering - coating method" to prepare polytetrafluoroethylene (PTFE) nanofiber membranes. First, polyethylene oxide (PEO) and PTFE are formulated into a spinning solution at a specific ratio, and a PTFE/PEO precursor nanofiber membrane is made by electrospinning machine electrospinning technology. Then, the precursor membrane is sintered to decompose the PEO carrier and leave the basic structure of the PTFE nanofiber membrane. Finally, a solution containing polyvinyl alcohol (PVA) is coated on the membrane surface, and an acetalization reaction is carried out with glutaraldehyde (GA) to construct a hydrophilic layer on the pore surface of the membrane. The entire process is continuous and efficient, ensuring the stability of the membrane's quality and performance.
(2) Significant Reduction in Membrane Pore Size
By adjusting the mass ratio of the spinning carriers PEO and PTFE, the optimization of the pore size of the PTFE nanofiber membrane was successfully achieved. When the PEO/PTFE mass ratio decreases, the viscosity of the spinning solution, the morphology of the nanofibers, and the micro - structure of the membrane change. As can be seen from the scanning electron microscope (SEM) images (Figure 2), with the decrease of the mass ratio, the nanofibers change from a spindle - shape to spherical beads, the fiber diameter becomes thinner, and the pore size of the membrane also decreases. Experimental data show that the average pore size decreases from 288.5 nm to 161.3 nm.

Figure 2. (a) Scanning electron microscope images of Mp1 - 4 and (b) Ms1 - 4; (c) Pore size distribution in Ms1 - 4.
(3) Efficient Filtration of Solid Particle Suspensions
The optimized PTFE nanofiber membrane exhibits excellent performance in filtering solid particle suspensions. Suspensions of carbon particles (1 μm) and SiO₂ particles (100 nm) were selected for the experiment. The results show that the membrane has a high rejection rate of 99.3% for carbon particles and 97.3% for SiO₂ particles. At the same time, the water permeation flux of the membrane has also been significantly increased, from 68.56 L·m⁻²·h⁻¹ to 1056.16 L·m⁻²·h⁻¹. This benefit from the optimized pore structure and hydrophilic modification, enabling the membrane to effectively intercept particles while ensuring a high water flux. (See Figure 3)

Figure 3. (a) Water contact angle, (b) pure water flux, (c) carbon particle separation efficiency of samples Ms1 - Ms4.
(4) Good Stability in Harsh Environments
The prepared PTFE nanofiber membrane has excellent stability in harsh environments. In the chemical stability test, the membrane was immersed in strong acid (HCl with pH = 2), strong base (NaOH with pH = 12), and high - concentration sodium hypochlorite (1000 ppm NaClO) solutions for 7 days. Although the water contact angle increased slightly, it still remained at a relatively low level, indicating that the hydrophilicity of the membrane could still be maintained in harsh chemical environments. In the wear - resistance test, after 100 friction cycles (with a 5 - g weight placed on 1000 - mesh sandpaper), there was no significant change in the macroscopic structure and performance of the membrane, reflecting good wear - resistance. (See Figure 4)
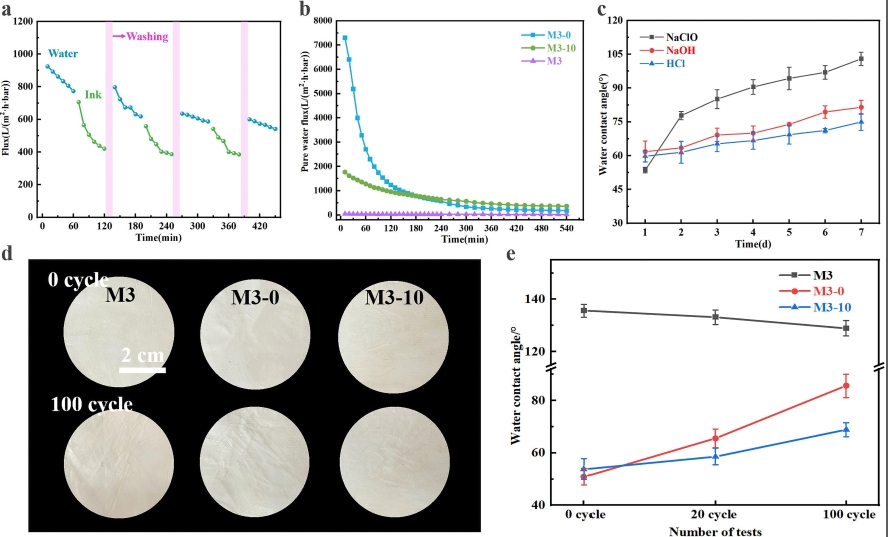
Figure 4. (a) Cyclic stability test of sample M3 - 10 for water and ink solutions at 0.05 MPa for 60 minutes each, followed by 10 - minute physical hydraulic washing; (b) long - term stability of samples M3, M3 - 0, M3 - 10; (c) WCA of sample M3 - 30 after immersion in HCl with pH = 2, NaOH with pH = 12, and 1000 - ppm NaClO solutions for 7 days; (d) digital images of M3, M3 - 0, M3 - 10 before and after 100 friction cycles; (e) WCA of M3, M3 - 0, M3 - 10 for 0, 20, and 100 friction cycles.
Polytetrafluoroethylene (PTFE) membranes have great potential for treating wastewater in harsh environments due to their excellent stability. However, the difficulties in pore structure optimization and functional modification of biaxially stretched PTFE membranes have limited their further application in liquid separation. Therefore, this study proposed a simple and efficient method for preparing electrospun PTFE nanofiber membranes and hydrophilic modification. This method optimizes the pore structure of PTFE nanofiber membranes by adjusting the mass ratio of the spinning carrier polyethylene oxide (PEO) and PTFE, and then introduces acetalized polyvinyl alcohol (PVA) on the pore surface of the membrane to improve hydrophilicity.
The average pore size changed from 288.5 nm to 161.3 nm, and the water contact angle decreased from 135.7° to 50.9°. This not only increased the water permeation flux from 68.56 L·m⁻²·h⁻¹ to 1056.16 L·m⁻²·h⁻¹, but also achieved high rejection rates of 99.3% and 97.3% for carbon (1 μm) and silica (100 nm) particles, respectively. At the same time, the obtained PTFE nanofiber membrane also exhibited excellent hydrophilic stability after long - term exposure to harsh environments (HCl with pH = 2, NaOH with pH = 12, 1000 - ppm NaClO) and 100 friction cycles (with a 5 - g weight placed on 1000 - mesh sandpaper). This method of preparing hydrophilic PTFE nanofiber membranes shows great potential in practical applications of wastewater treatment.
Article Source: https://doi.org/10.1016/j.seppur.2024.129494