Copyright © 2022 Foshan MBRT Nanofiberlabs Technology Co., Ltd All rights reserved.Site Map
1 Introduction
In recent years, the problems of energy crisis and environmental pollution have become more serious, and the production of sustainable, renewable and clean energy is imminent. Electrocatalysis technology can realize clean and renewable energy conversion. Chemical composition, surface condition, and microstructure of nanomaterials determine the electrocatalytic activity. The electrospinning fibers possess the characteristics of easy preparation, good porous structure and uniform distribution. The carbonized nitrogen-doping carbon nanofibers have become one of the hot research topics thanks to their unique pore structure, high surface area and excellent conductance properties. Besides, the obtained carbon nanofibers have abundant source materials and wide application in electrocatalysis .
This review mainly introduces the preparation and morphology characteristics of four different fiber structures, including porous structure, core-shell structure, hollowstructure andmulti-channel structure. Then the application progresses of nitrogen doped carbon nanofibers in the fields of oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER) and carbon dioxide reduction reaction (CO2RR) are reviewed. Finally, the future development of carbon nanofibers prepared by electrospinning is summarized.
2. Structure of electrospun nitrogen-doped carbon nanofibers
2.1. Porous structure
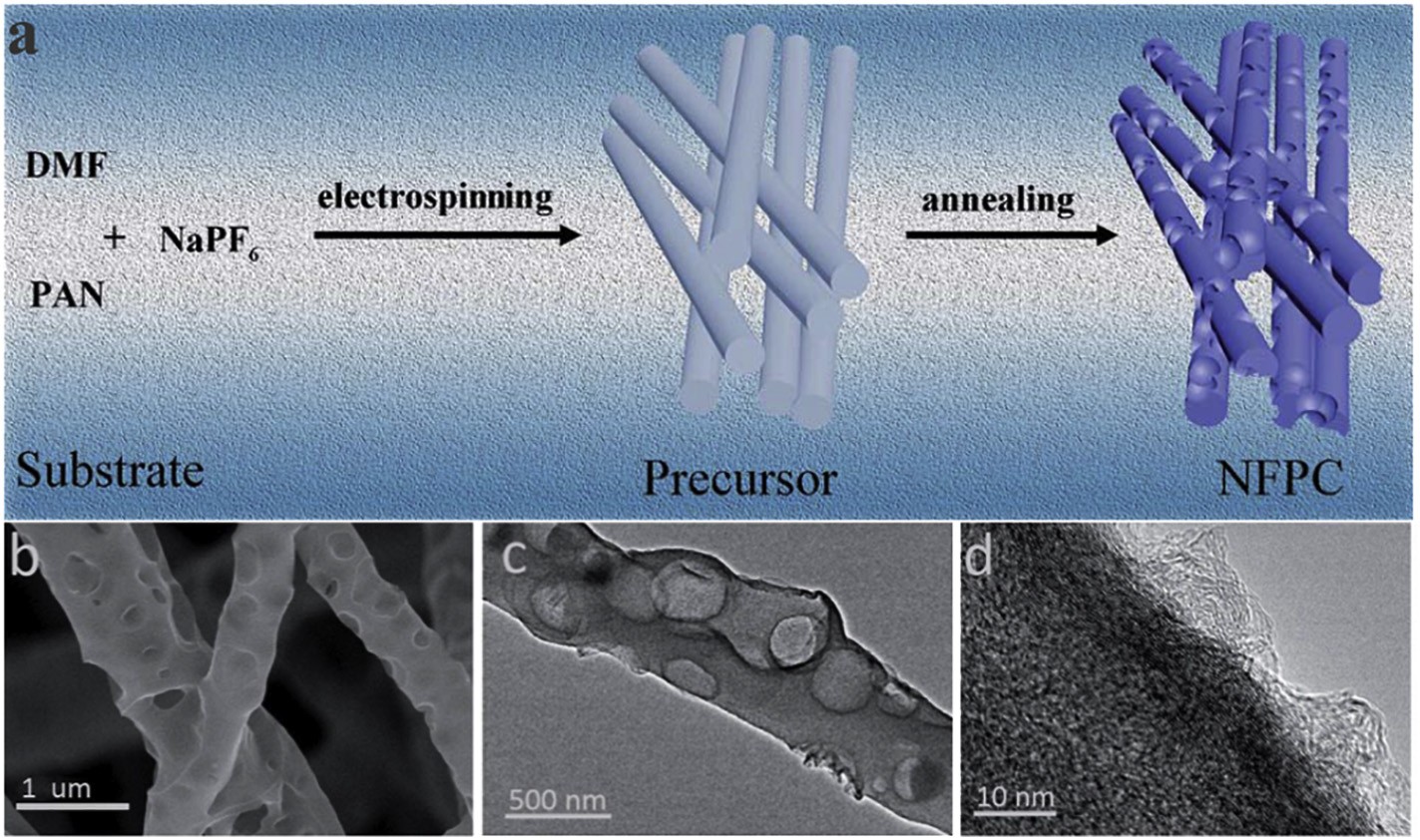
Fig 1 (a) The synthesis process of N, F, P ternary doped macro-porous carbon fibers. (b-d) TEM of the as-synthesized NFPC
Although the nitrogen-doped carbon fibers produced by electrospinning have high yield and excellent conductivity, the pore structure needs to be further improved to expose the active sites and facilitate electron transferring. Adding the appropriate amount of templates to the precursor solution can produce pores on the surface of fibers and produce porous carbon nanofibers .Moreover, the pore size can be selectively adjusted by adding template agents of different sizes and subsequent processing. In carbon-based catalysts, excellent and stable nanostructures are essential for the accessibility of active sites. Micropores are beneficial to the accessibility of the active center and the reversible adsorption/desorption of gas on the catalyst surface in the electrocatalytic process . Furthermore, macropores and mesopores usually also play important roles in mass transferring . Therefore, it is highly desirable to apply porous structures in carbon fibers for electrocatalysis.
2.2. Core-Shell structure
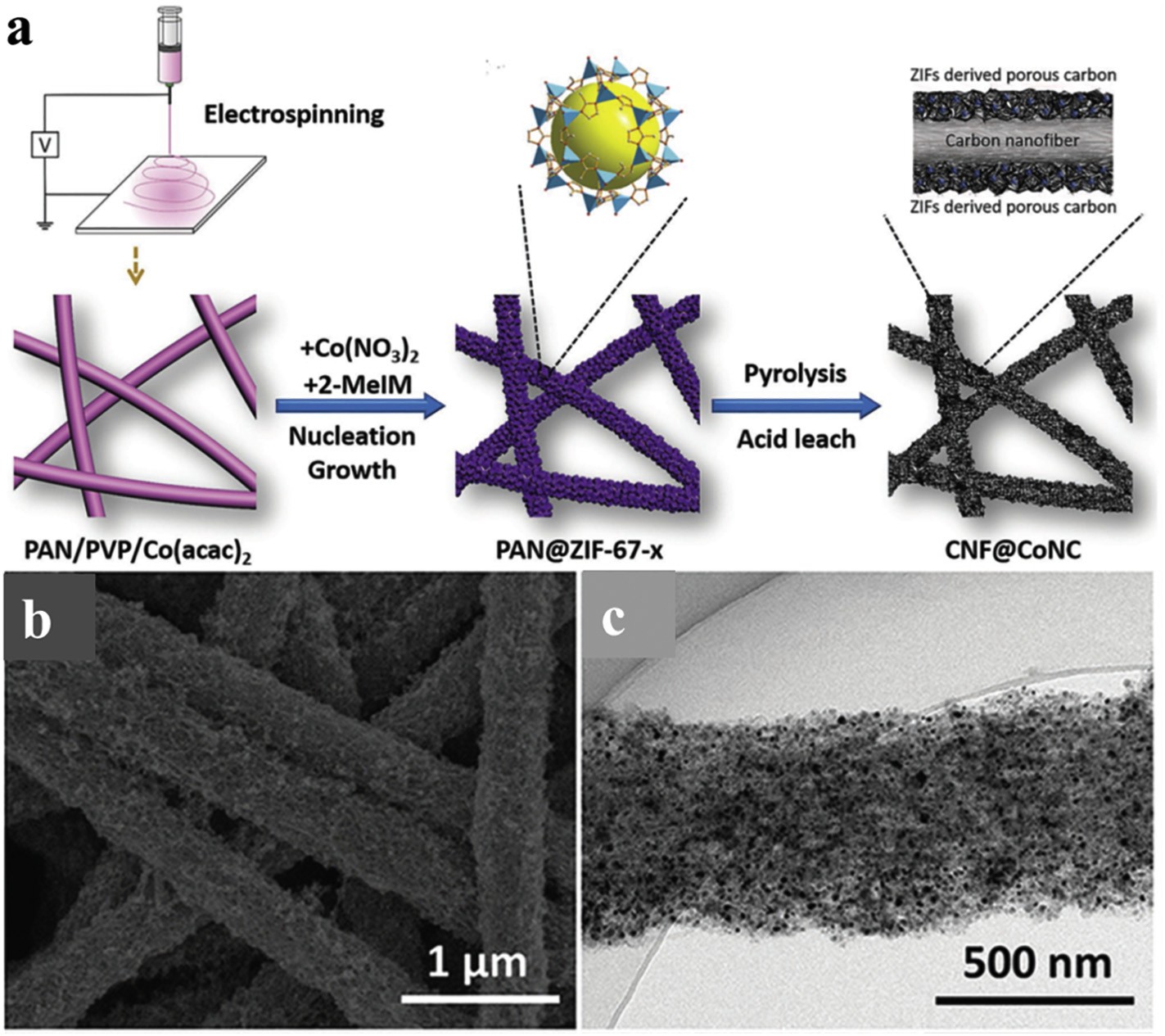
Fig 2 (a) Schematic illustration of the synthesis of heteroatom-doped carbon nanofiber derived from core–shell PAN@ZIF-67 fiber. (b) SEM image and (c) TEM image of CNF@Zn/CoNC
In recent years, metal@carbon (M@C) nanocomposites have become functional nanomaterials with excellent electrocatalytic activity for a series of reactions (such as ORR, OER, HER and CO2RR) . For M@C catalyst with core-shell structure, the carbon shell can protect the metal core from corrosion in extreme electrolyte, the electrons metal core can penetrate from the carbon shell and participating in electrocatalytic reaction on the surface of carbon shell. Moreover, the interfacial charge transfer from themetal core to the carbon shell controlled the electronic interaction. The heteroatoms doping in carbon structure further improve the synergy between core and shell . Based on above mentioned factors, nitrogen-doped carbon nanocomposite fibers with core-shell structure are widely prepared and explored as electrocatalysts.
2.3. Hollow structure
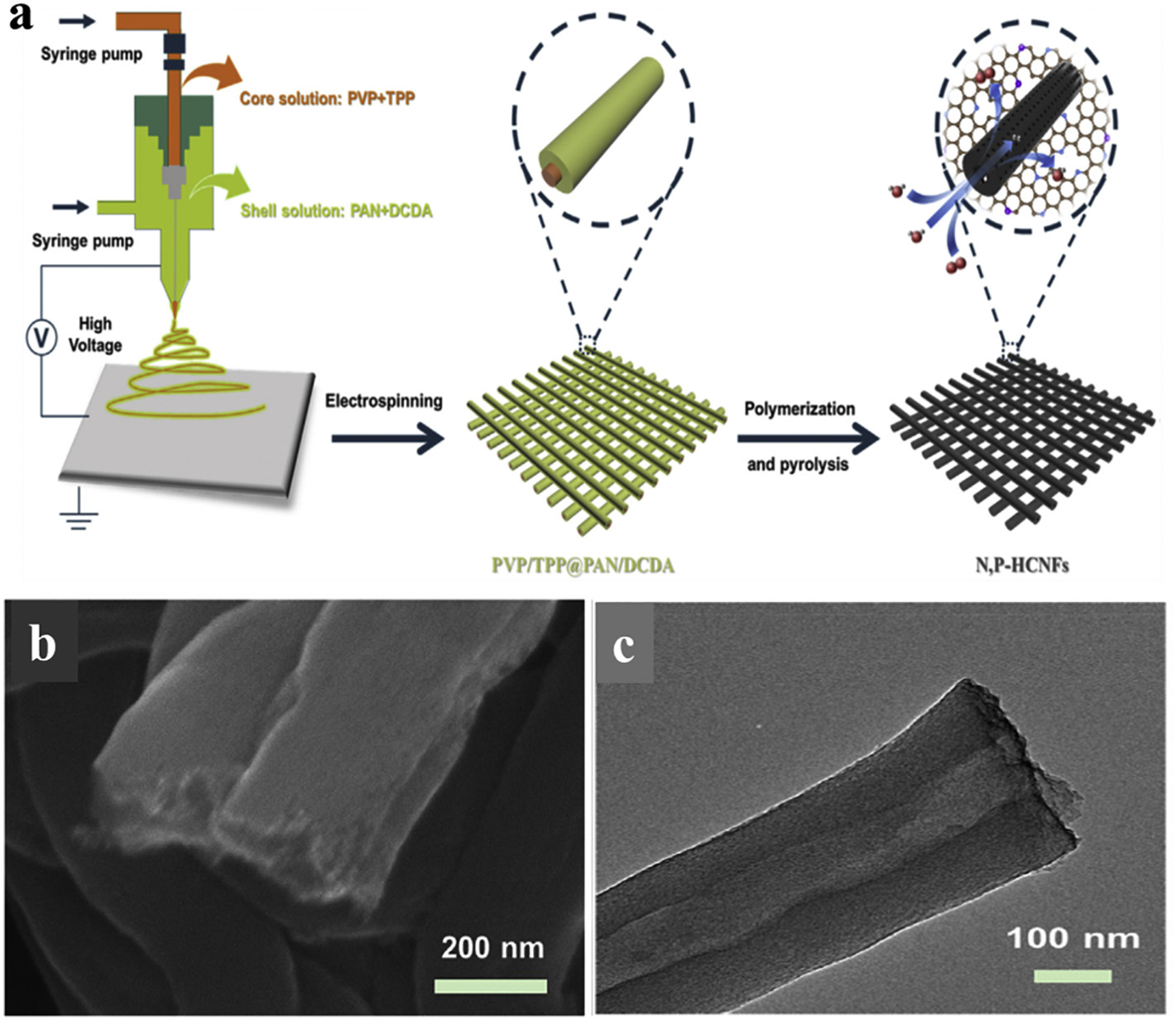
Fig. 3. (a) Schematic representation of the fabrication procedures towards N, P-HCNFs. (b) SEM image of fractured surfaces of N, P-HCNFs. (c) TEM image of N, P-HCNFs
At present, it is still an arduous challenge to construct suitable model materials with considerable active sites and superiormass transfer performance, as well as to study the effect of mass transfer on electrocatalytic activity. Specifically, carbon hollow nanostructures provide a unique platform owing to their unique structural features (such as enlarged contact area, valid ion diffusion pathway and continuous electronic transmission) to improve mass transfer performance, thereby enhancing the number of active sites. Therefore, the preparation of hollow carbon fibers can effectively improve the electrocatalytic performance.
2.4. Multi-channel structure
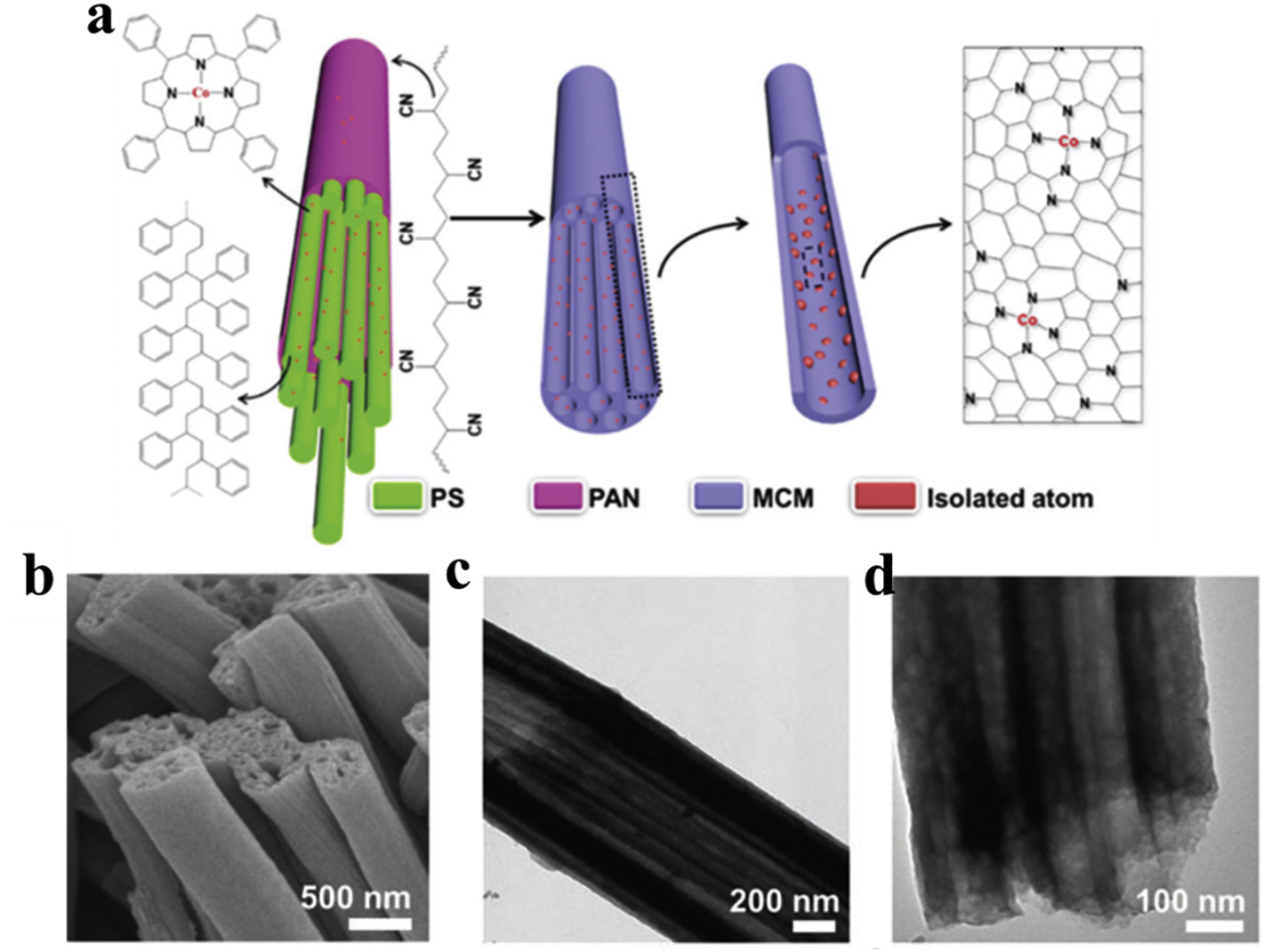
Fig. 4. (a) Schematic illustration of the synthetic process of Co@MCM. (b) FESEM image of Co@MCM. (c-d) TEM images of Co@MCM
In short, multi-channel structure can well expand the specific surface area and expose the active sites. It possesses good electron/mass transfer ability and achieving high electrocatalytic activity. Moreover, when preparing metal single-atom electrocatalysts, a multichannel carbon matrix with high conductivity and a highly open structure can effectively promote mass transfer and charge transfer, and inhibit nanoparticle aggregation. When preparing electrocatalysts with metal nanoparticles, a large number of metal nanoparticles are uniformly dispersed on the inner and outer surfaces of multichannel carbon fibers, which provides effective active sites, inhibits the loss of active substances and ensures the cycle stability of the catalyst.
3. Electrocatalytic applications
The carbon nanofibers prepared by electrospinning have attracted the attention of many scholars as low-cost, environmentally friendly and efficient electrocatalysts with large surface area. However, conventional pure carbon nanofibers usually exhibit insufficient catalytic activity. Activated by heteroatom dopants, nitrogen-doped carbon nanofibers aremore active for electrochemical reactions, and their electrocatalytic performance is similar or even better to Pt based electrocatalysts. This review mainly summarizes the research reports of nitrogen-doped carbon nanofibers in ORR, OER, HER, CO2RR.
3.1. Oxygen reduction reaction
Themechanism of oxygen reduction reaction is that oxygen molecules form products by accepting electrons. ORR is one of the key reactions in plenty significant energy conversion devices (metal-air batteries, fuel cells, etc.) and certain industrial processes. However, ORR hinders the practical application of fuel cells and metal-air cells due to its intrinsic sluggish electrode dynamics. So far, platinum-based materials have been developed as the most efficient catalysts for ORR, but many scholars have focused on finding alternative catalysts because of high cost, resource shortages and low stability of Pt. As an alternative, carbon nanofibers prepared by electrospinning have gained widespread attention due to their good electronic conductivity and large specific surface area.
3.2. Oxygen evolution reaction
Oxygen evolution reaction is critical for renewable energy conversion and storage equipment, such as water electrolysis devices and rechargeable metal-air batteries. Nevertheless, the OER has sluggish kinetics due to the involvement of a four-electron transfer reaction, which requires the removal of four protons from water molecules to produce an oxygenmolecule. Therefore, it is urgent to explore highly efficient electrocatalysts to reduce the kinetic barrier of the reaction, and hence the overpotential (η) of the OER. To date, precious-metal oxides, such as RuO2 and IrO2, are considered as the state-of-the-art electrocatalysts for the OER. However, their stability is limited and the cost is high, so it is of prime importance to explore other effective and stable OER electrocatalysts with inexpensive and abundant sources.
At present, transition metal alloys, oxides, hydroxides, nitrides, phosphides, carbides, etc. have been applied to prepare OER electrocatalysts. Among them, transition metal nitrogen-doped carbides have received much attention due to their outstanding properties.
3.3. Hydrogen evolution reaction
At present, non-noble metal catalysts are widely studied as alternatives. Among them, carbon materials doped with transition metals and heteroatoms have attracted much attention due of their excellent performance. Nitrogen dopants enhanced H⁎ adsorption, promoted the interaction between the electrolyte and the electrode, and achieved effective mass and charge transfer. As mentioned above, controlling the process parameters of electrospinning could produce carbon nanofibers with the desired pore size andmorphology, which is very suitable for hydrogen storage.
3.4. CO2 reduction reaction
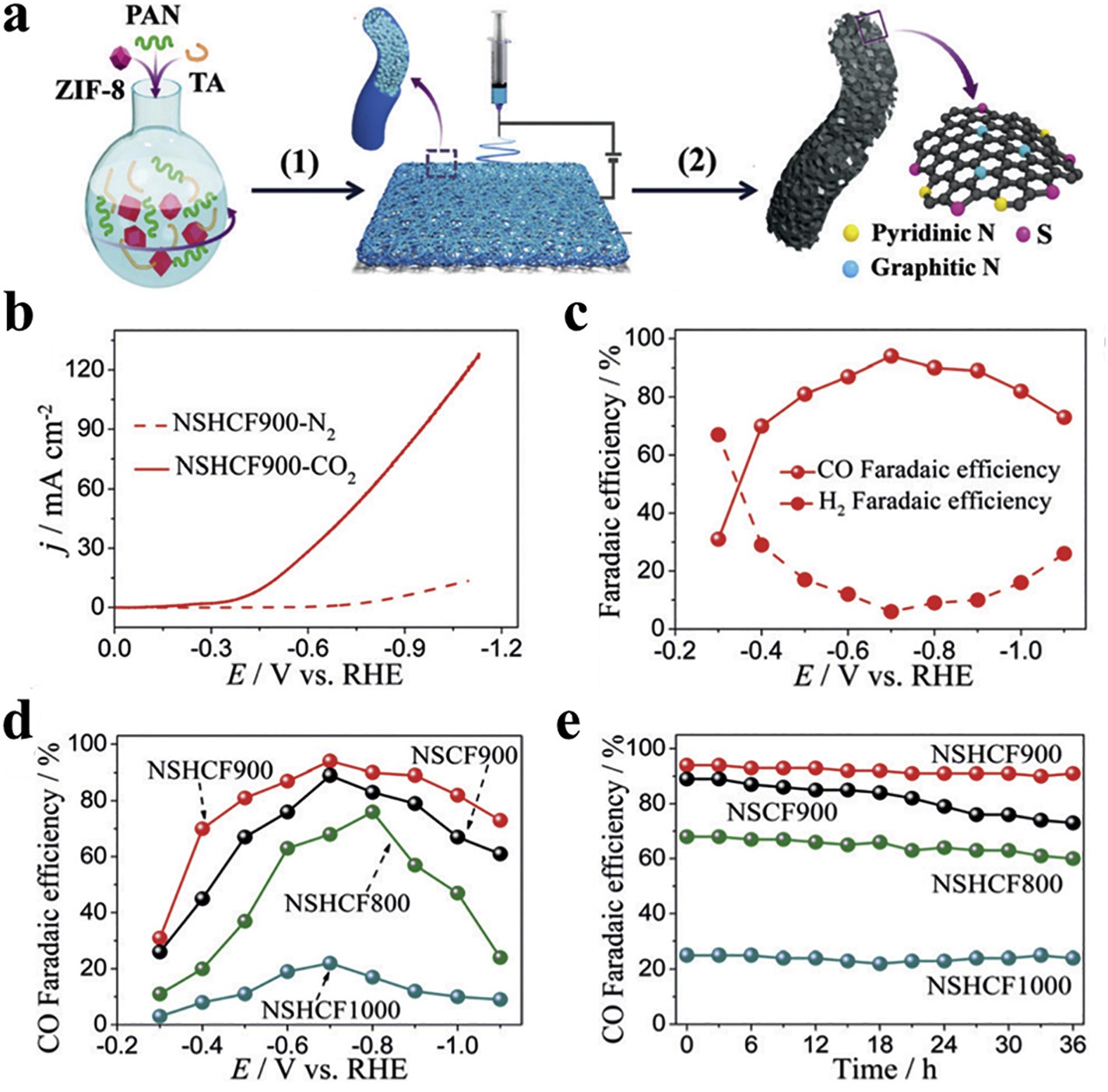
Fig. 5 N and S co-doped, hierarchically porous carbon nanofiber (NSHCF)materials
Nevertheless, preciousmetal resources such as Pt are in short supply, and it is feasible to use transition metalmaterials or heteroatom-doped metal-free materials as CO2RR electrocatalysts. Pan et al. used electrospinning technology to prepare N and S co-doped, hierarchically porous carbon nanofiber (NSHCF) materials. As shown in Fig.5 the fiber membrane has certain flexibility and independence, and can be directly used as a cathode. It is a high-efficiency CO2RR electrocatalyst which only needs about 1.2mg of catalyst loading to obtain CO with a Faraday efficiency of 94% and a current density of −103 mA cm−2. Meanwhile, as shown in Fig. 5e, NSHCF900 has favorable stability,which is very critical for its practical application.