Copyright © 2022 Foshan MBRT Nanofiberlabs Technology Co., Ltd All rights reserved.Site Map
1. The production of NH3
Ammonia, as an important fertilizer, is one of the most basic chemicals to maintain human life. The transformation of rich N2 on earth into NH3 remains a challenge because it has chemical inertia, high bond energy and permanent dipole in N≡N triple bond. The industrial scale ammonia synthesis is mainly operated by Haber-Bosch method, which depends on the iron based catalyst and high temperature and high pressure conditions. In addition, this process requires a large amount of energy consumption and a large amount of greenhouse gas. It is still an elusive scientific and technological goal to develop sustainable technology to produce NH3 under mild conditions. Up to now, the development of NRR (electrochemical nitrogen reduction) catalysts has been focused on precious metals, transition metals and their compounds. However, due to the weak adsorption of nitrogen, the activation of nitrogen is still limited by transition metals. At the same time, the d-track in transition metal also participates in the competition, which leads to the reduction of Faraday efficiency of NRR.
In this article, the researchers have proposed a breakthrough in the concept and experiment. The high energy barrier of N2 absorption and activation can be overcome by adjusting the center position of boron in S-B/CNFs. The effects of boron as electronic acceptors and sulfur and phosphorus as electronic donors on NRR activity were studied theoretically and experimentally.
2. The preparation of nanometer carbon fiber material
As a proof of concept experiment, a series of boron, sulfur and phosphorus doped / Co doped carbon nanofibers with different content of boron, sulfur and phosphorus were successfully designed and synthesized. The typical synthesis process of synthetic materials is shown in Figure 1. Firstly, H3BO3 was dissolved in Polyacrylonitrile solution to obtain homogeneous precursor solution. Then, pan / H3BO3 nanofiber membranes were prepared by electrospinning. Pan / H3BO3 nanofiber films were transformed into B-doped carbon nanofibers (B / CNFs), S-B Co doped CNFs (S-B / CNFs) and P-B Co doped CNFs (P-B / CNFs) by the graphitization process of sulfur vapor or phosphorus vapor treatment, respectively.
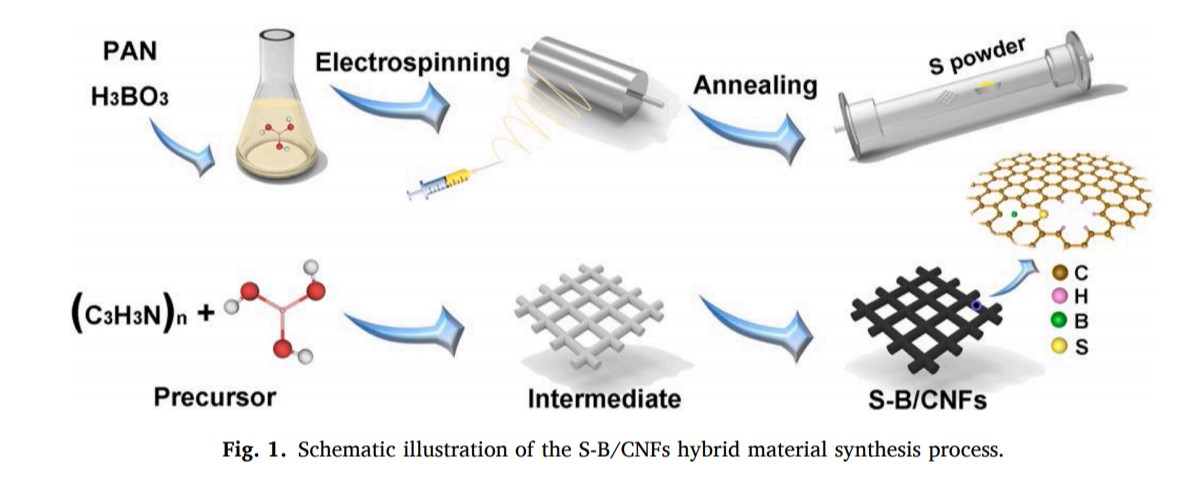
Fig.1. Schematic illustration of the S-B/CNFs hybrid material synthesis process.
3. The nano fiber surface properties
The structure and morphology of the materials were studied by field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM). B8.09/CNFs film is a three-dimensional network composed of randomly assembled nanofibers with an average diameter of about 200nm, which is hydrophilic. The morphology and structure of P4.12-B8.09/CNFs Co doped with P and B also show that the three-dimensional nanofiber network is hydrophobic surface. After s-steam treatment, the synthesized S6.23-B8.09/CNFs (Fig. 2) showed hydrophilic surface, indicating that s doping initiated the transition of surface properties from hydrophobicity to hydrophilicity.
The results show that the surface properties of B/CNFs can be changed by different doping elements. The hydrophilicity of S6.23-B8.09/CNFs is very important for NRR. Because compared with hydrophobic surface, more potassium ions adsorb and gather on the hydrophilic electrode surface. The relatively high concentration of potassium ions on the electrode will strongly hinder the proton migration from the solution to the electrode surface, thus inhibiting competitive HER (hydrogen evolution reaction of electrocatalyst).
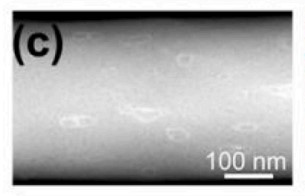
Fig.2. hydrophilic surface
4. Carbon nanotubes NRR activity
In order to study the effect of boron, sulfur and phosphorus doping on the NRR activity of carbon nanotubes, a series of B / CNFs with different boron content were designed. It was found that the yields of Fe and NH3 in carbon nanotubes varied with the B content. P-B / CNFs with P content of 4.12at% and B content of 8.09at% had the highest NRR activity. In other words, the S content of S-B / CNFs hybrids also affected NRR activity. Therefore, when the boron content in carbon fiber is ideal, the content of secondary phosphorus and sulfur has a significant effect on NRR activity.
According to the theory of P-band center, the catalytic activity of nonmetallic elements is related to the central position of pz orbital. Therefore, as shown in Figure 3, the researchers also plotted the proposed density of states of boron atoms in the 2-S-B / CNFs model. In order to study the reaction pathway and mechanism of excellent NRR activity on S6.23-B8.09/CNFs hybrid materials, density functional theory (DFT) was used to calculate the P-band center of B, and the energy distribution map of NRR on S6.23-B8.09/CNFs hybrid materials was drawn. In the chemical structure of S6.23-B8.09/CNFs, S can only form double bond with C, so S atom can only form at the edge of carbon skeleton. The results show that the BC3 structure is the main active center of NRR. In this work, the introduction of S atom into B-doped carbon structure will lead to local electron distribution and charge conversion, which is the key factor to determine the NRR activity.
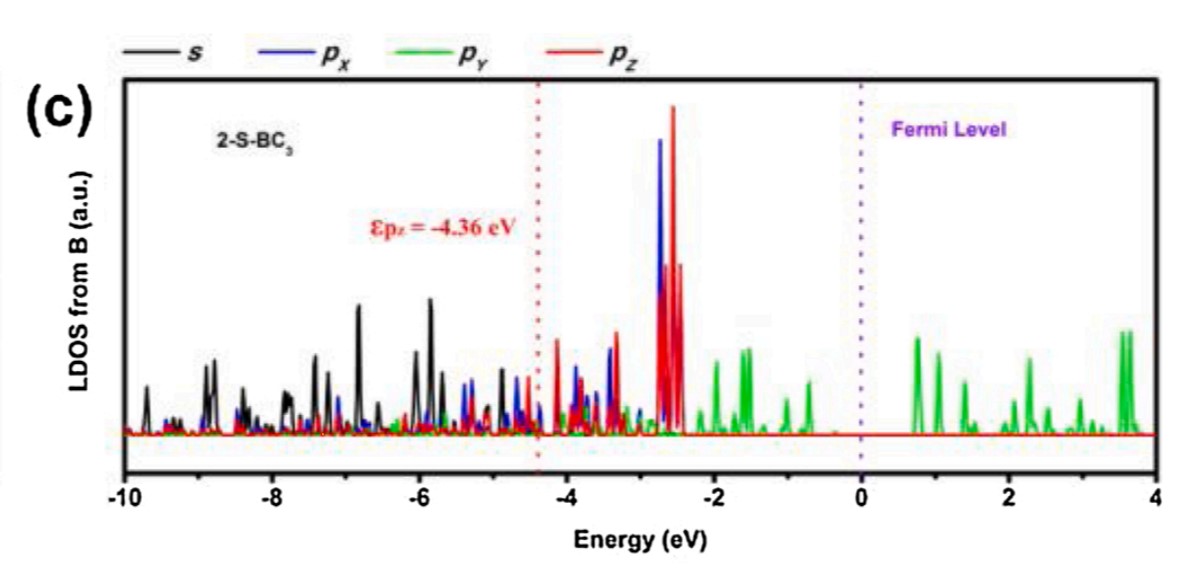
Fig.3 The proposed density of states of boron atom
5. The stability of the nanofibers
Stability is very important for practical application. Figure 4 shows a 10 hour cycle of S6.23-B8.09/CNFs for NRR in the -0.7v and Rh (0.5m K2SO4) electrolytes. Fe of NH3 and NH3 remained stable for 10 consecutive hours. The yield of Fe and NH3 did not decrease significantly, indicating that S6.23-B8.09/CNFs had good repeatability. Figure 5 shows the FE-SEM, TEM and HRTEM images of S6.23-B8.09/CNFs after 10h electrochemical stability test, indicating that the morphology of one-dimensional fiber remains unchanged compared with the original catalyst.
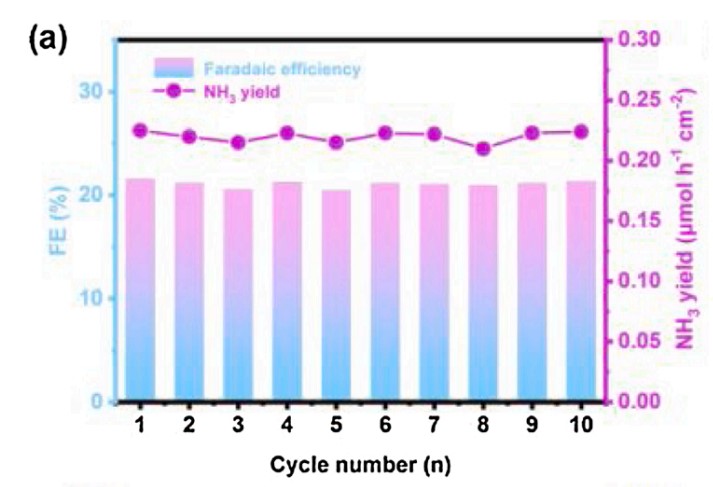
Fig.4 The yield of Fe and NH3.
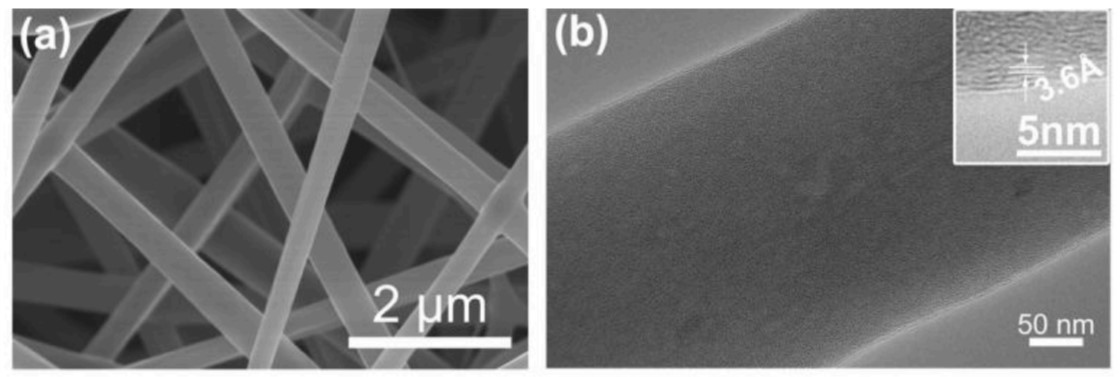
Fig.5FE-SEM, TEM and HRTEM images
6. Carbon nanotubes in S, B, doping effects on its ehrs activity
In order to understand the effect of S and B doping on HER activity, the Gibbs free energy of hydrogen adsorption was calculated. The results show that the B active sites of B / CNFs and 2-S-B/CNFs have similar hydrogen absorption capacity (1.14 EV and 1.13 EV). However, the S-active site of 2-S-B / CNFs model has a weak hydrogen absorption capacity (1.95 EV). Therefore, the introduction of S-site in the 2-S-B / CNFs model will suppress her, indicating that S, B Co doped CNFs show enhanced NRR performance. Through the above reaction pathways and free energy analysis, the 2-S-B / CNFs model has ideal NRR performance.
7. Conclusion
In conclusion, a series of boron, sulfur or boron, sulfur Co doped carbon materials were synthesized in a nano reactor with carbon nanotubes as the main body, and the content of boron, sulfur and phosphorus in carbon nanotubes was controlled. The well-defined S6.23-B8.09/CNFs showed excellent NRR performance. The NH3 yield was as high as 0.223 μ mol h − 1 cm-2, and the Faraday efficiency was 22.4% at − 0.7V. Compared with the reversible hydrogen electrode in 0.5 m K2SO4, the hybrid of B8.09/CNFs and P4.12-B8.09/CNFs was superior. The heteroatom doping of S atom causes the change of the pz orbital center of B, which is beneficial to the adsorption of N2 on S-C-B site and reduces the energy barrier of the first protonation reaction rate determining step. The relationship between P-band center and NRR activity of heteroatom doped carbon catalyst was revealed by calculation and experiment, which laid a foundation for its further application.
Reference:Wen Yankun et al. Metal-free boron and sulphur co-doped carbon nanofibers with optimized p-band centers for highly efficient nitrogen electroreduction to ammonia[J]. Applied Catalysis B: Environmental, 2021, 292
Paper link :
https://doi.org/10.1016/j.apcatb.2021.120144