Copyright © 2022 Foshan MBRT Nanofiberlabs Technology Co., Ltd All rights reserved.Site Map
Catalog:
1. Properties of PLLA materials
2. Application of PLLA nanofibers in tissue engineering
3. Experimental parameters of electrospun PLLA
4. Common problems and solutions
1. Properties of PLLA materials
Since there is an asymmetric carbon atom in the lactic acid molecule, which has optical activity, polylactic acid can also be divided into dextral polylactic acid (PDLA), left-handed polylactic acid (PLLA), racemic polylactic acid (PDLLA), and non optical polylactic acid (Meso PLA). PLA is an important biodegradable polymer material, characterized by non-toxic, non irritating, biodegradable absorption, high strength, good plasticity, and easy processing and molding. The degradation cycle is 2~12 months.
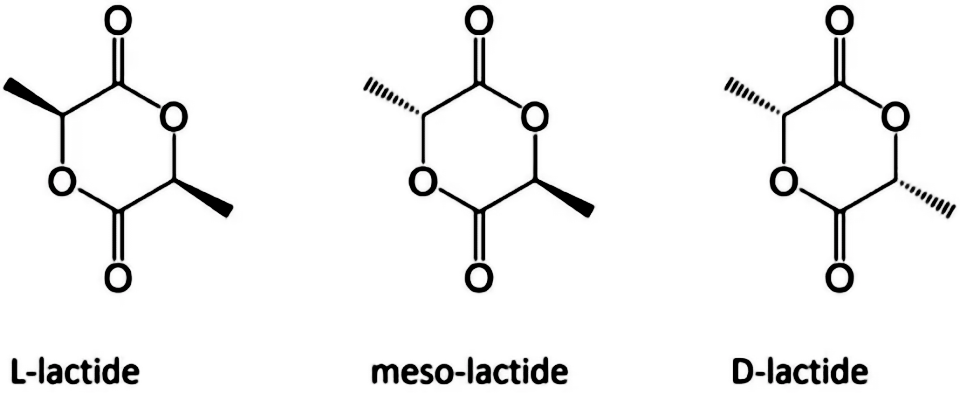
Fig. 1 Enantiomeric form of lactic acid
2. Application of PLLA nanofibers in tissue engineering
PLLA is one of the first synthetic polymer materials recognized as suitable for tissue engineering due to its non-toxic, non irritating, biodegradable absorption and high strength. The nanofiber scaffold produced by the invention can be used for specific tissue engineering applications, including bone, cartilage, blood vessel and skin tissue regeneration.
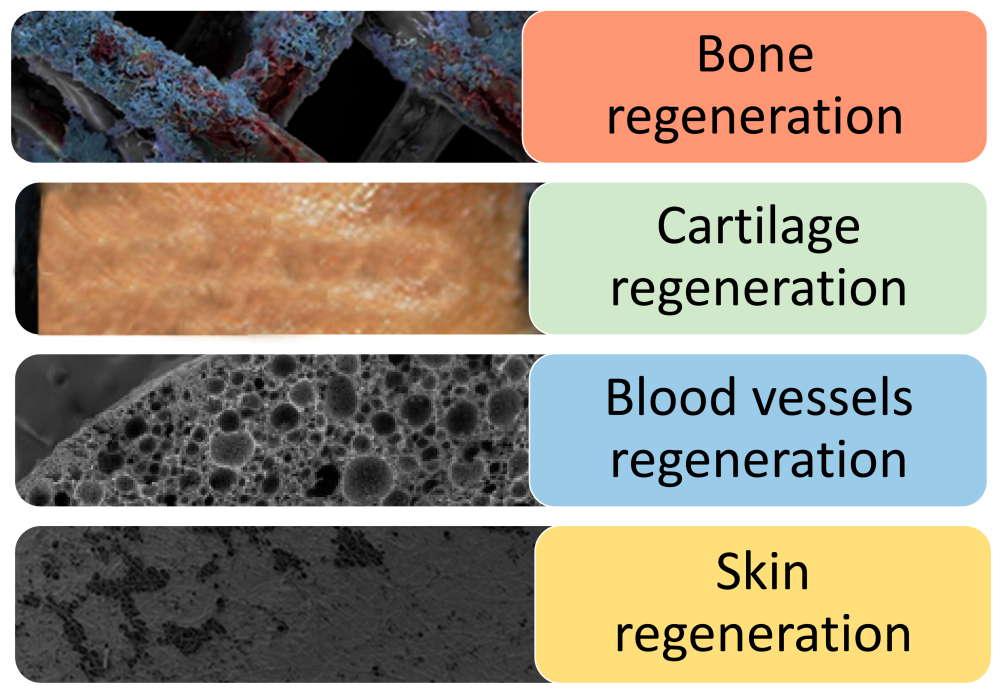
Fig. 2 Application of PLLA stent in tissue engineering
(1) Bone tissue
In bone tissue engineering, electrospinning has been widely used to prepare nanofiber scaffolds, whose structure is close to the nano collagen fibers of bone. In actual preparation, electrospun PLLA scaffold will be prepared in combination with surface modification to guide cells to differentiate into bone lineage and achieve the best bone regeneration performance.
A research team successfully modified the surface of PLLA nanofibers prepared by electrospinning through the surface deposition of osteogenic ECM. These scaffolds were then used to detect the response of mouse bone marrow stromal cells after implantation of the scaffold. The results showed that compared with pure PLLA nanofibers, the mineral growth, ALP activity and cell morphology of the modified structure were in the best state.
(2) Cartilage tissue
Although PLLA nanofiber scaffolds have shown significant effects on cartilage repair, their fine fibers lead to limited bearing capacity.
PLLA nanofiber scaffold made by phase separation and pore forming agent leaching has been proved to be the optimal scheme for cartilage repair in vivo and in vitro. This is due to the high porosity and interoperability of these scaffolds, as well as their good degradability. In addition, the appropriate size of the scaffold hole also improves the functional characteristics of cartilage, thus providing some different solutions for the regeneration and repair of cartilage tissue.
(3) Vascular tissue
Recently, a research team has made multi-layer scaffolds for vascular tissue engineering: PCL, collagen and PLLA nanofibers are used to simulate the inner membrane, middle membrane and outer membrane respectively. These nanofibers were made into three-layer tubular scaffolds by continuous electrospinning, and cultured endothelial cells and smooth muscle cells for biological activity evaluation. The final evaluation experiment results showed that the collagen in the middle layer significantly improved the adhesion and proliferation of SMC, and the proliferation of endothelial cells increased significantly with culture, indicating the non cytotoxicity of the construct.
(4) Skin tissue
In dermal fibroblasts culture in vitro, the mixed scaffold induced higher cell seeding efficiency and improved fibroblast adhesion and proliferation compared with collagen sponge. On the other hand, in vivo wound healing evaluation showed that the composite scaffold healed faster and more effectively than the collagen scaffold. In most cases, PLLA, collagen and gelatin are often combined to produce nanofiber scaffolds, which show physical and biological characteristics matching with skin substitutes.
PLLA is also used to produce multilayer scaffolds that are very similar to natural skin structures. A research team has developed a new type of double-layer scaffold, which is composed of chitosan/PCL nanofiber pad on the surface and PLLA microporous disk on the bottom. In this work, keratinocytes and fibroblasts were cultured as epidermal equivalent and dermal equivalent respectively. The final results showed that the cell proliferation in the double-layer scaffold was higher than that in the single-layer CP nano pad and PLLA microdisk. In addition, the evaluation of genes and proteins showed that the wound healing was active, which again confirmed that the double-layer scaffold could provide a suitable microenvironment for stimulating skin regeneration.
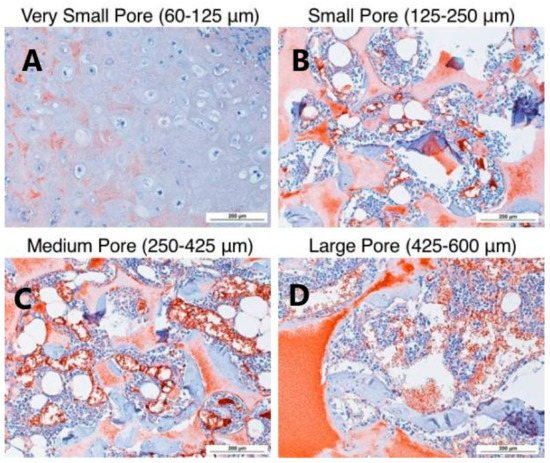
Fig. 3 Small Aperture (125-250 μ m) In vitro cartilage differentiation of human bone marrow mesenchymal stem cells induced by β - lactamase scaffold Large specific aperture (425-600 μ m) The stents in our body better support cartilage formation.
Cited by: Capuana, E; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. https://doi.org/10.3390/polym14061153
3. Experimental parameters of electrospun PLLA
3.1 Experimental parameters for preparation of poly (L-lactide)/hydroxyapatite (PLLA/HA) nanocomposite scaffold
Material configuration: Dissolve 1g of PLLA (intrinsic viscosity 0.90-1.20dL/g) in 5mL of dichloromethane, and disperse 0.1g of nano HA powder (particle size<200nm) in the polymer solution under stirring, and transfer the obtained suspension to the medical syringe.
Electrospinning experiment parameters: positive voltage 15kV, liquid supply speed 1.5mL/h, needle 23G, spinning distance 15cm.
Reference: Rainer A, Spadaccio C, Sedati P, et al. Electrospun Hydroxapatite Functionalized PLLA Scaffold: Potential Applications in Sternal Bone Healing [J] Annals of Biomedical Engineering, 2011, 39(7):1882-1890.
3.2 Experimental parameters for preparation of PLLA/PCL oriented fiber scaffold
Material configuration: PLLA is dissolved in chloroform first, then in dimethylformamide (DMF) (4.25:0.75), while PCL is dissolved in chloroform/DMF (8:2). They were stirred for 3 hours, and then the polymer solution was loaded into the syringe by applying a positive voltage between the needle and the collector. The solution droplets leave the needle to form nanofibers and deposit at the same time. The percentage of PLLA/PCL is 47/53 wt%. The needle tip is placed 15 cm from the collector for PLLA and 20 cm for PCL, while rotating the disk at a linear rate set at 2800 rpm to collect parallel nanofibers.
Electrospinning test parameters: 5ml plastic syringe, 21 # needle, positive voltage (18 kV for PLLA, 24 kV for PCL)
Reference: Mashhadikhan M, Soleimani M, Parivar K, Yaghmaei P. ADSCs on PLLA/PCL Hybrid Nanoscaffold and Gelatin Modification: Cytocompatibility and Mechanical Properties Avicenna J Med Biotechnol. 2015 Jan-Mar; 7(1):32-8. PMID: 25926950; PMCID: PMC4388888.
3.3 Experimental parameters for preparation of pure PLLA nanofibers
Material configuration: dissolve PLLA in hexafluoroisopropanol, with the mass fraction range of 8%~10%, 20G (1/2 inch, flat mouth) stainless steel needle, the needle is connected to the positive pole of high-voltage power supply, and the receiving roller is connected to the negative pole of high-voltage power supply
Electrospinning experiment parameters: the liquid supply speed is 0.5~3mL/h (depending on the electrospinning process), the roller speed is 300 rpm, the slide travel is 20cm, and the spinning voltage is positive 15kV and negative 5kV
4. Common problems and solutions
(1) The boiling point of the solvent is low, so it cannot be spun at high temperature to avoid the blockage of the needle tip due to the rapid evaporation of the solvent.
(2) The solution shall be prepared by gradually adding particles or powders to prevent the dissolution rate from decreasing due to particle agglomeration and suspension.